
A technique to produce large quantities of anti-cancer immune cells from iPS cells
T cells are a type of immune cell found in the human body with the ability to detect and eliminate cancer cells. The method of using the patient’s own T cells, which are cultured outside the body and then returned to fight the cancer, has started to be applied. However, preparation of the T cells is a lengthy process. If T cells could be prepared in large quantities in advance, it would be possible to reduce the time needed for this therapy. T-CiRA has been working to develop a technique for safely culturing T-cells in large quantities using iPS cells.
The significance of producing T cells from iPS cells
Chimeric antigen receptor (CAR) T-cell therapy is currently approved in Japan for treating some blood cancers In this therapy, T cells are taken from the patient’s body, and a molecule that acts like a keyhole, recognizing and binding to a specific target (key) of cancer cells, is introduced. The modified cells are then cultured and returned to the patient’s body. The therapy is highly effective, but preparing the T cells requires a certain amount of time and is very costly. Reducing the time and cost needed for T cell preparation will be an important part of developing similar therapies for other cancers as well.
Some people might suggest culturing T cells in advance rather than using the patient’s own cells. The problem is that the properties of T cells change if they are cultured for too long, so it is not a realistic proposition. However, there are hopes for a method in which iPS cells are turned into T cells during culture, as it should be reasonably possible to safely prepare “fresh” T cells using this method.
However, conventional methods of turning iPS cells into T cells during culture are inefficient, and cells known as “feeder cells” also have to be used to facilitate cell differentiation and proliferation (Fig. 1a). Since feeder cells are obtained from animals, the cells could cause contamination with known or unknown viruses, etc., and feeder cells of the quality that could be used for medical applications are either extremely expensive or simply do not exist. We therefore set out to develop a method for culturing large quantities of T cells without the need for feeder cells.
We investigated a number of proteins and compounds believed to be important for the differentiation and proliferation of T cells, and we found that adding a certain protein and a certain compound to the culture medium dramatically increased the efficiency of the culture (Fig. 1b). After three weeks of culture, we obtained over 10 times more T cells than when nothing was added to the medium.
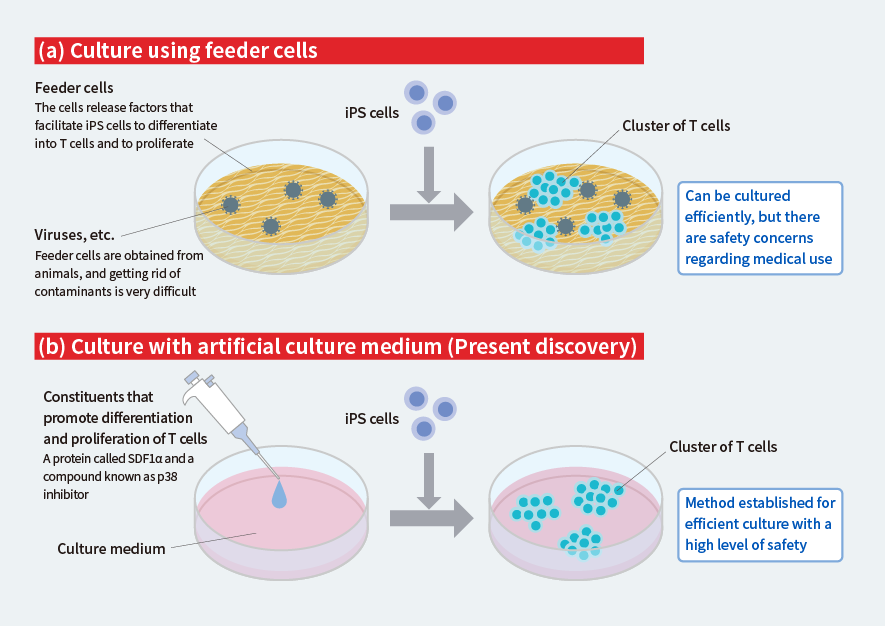 Figure 1. Difference between the conventional culture method and the new method
Figure 1. Difference between the conventional culture method and the new method
This method uses no animal-derived feeder cells, and, therefore, there is no risk of contamination with viruses by those cells, meaning highly safe T cells can be obtained.
Confirmation that the generated T cells work properly
Next, we examined whether the T cells created in this way retain the ability to detect and eliminate cancer cells. First, we introduced a protein that acts as a keyhole to identify a type of cancer called Wilms tumor 1 (WT1) into iPS cells, and then differentiated the iPS cells into T cells and made them proliferate using the culture method established above (Fig. 2, top). At the same time, we artificially created this cancer in mice. The proliferated T cells were administered to the mice with cancer, and the way the cancer changed and their lifespan were compared to those of mice that had not received the T cells.
Growth of the cancer was suppressed and the lifespan was longer in the mice that had been administered the T cells than in the untreated mice (Fig. 2, bottom). This confirmed that the T cells produced from iPS cells properly retained the ability to recognize and eliminate cancer.
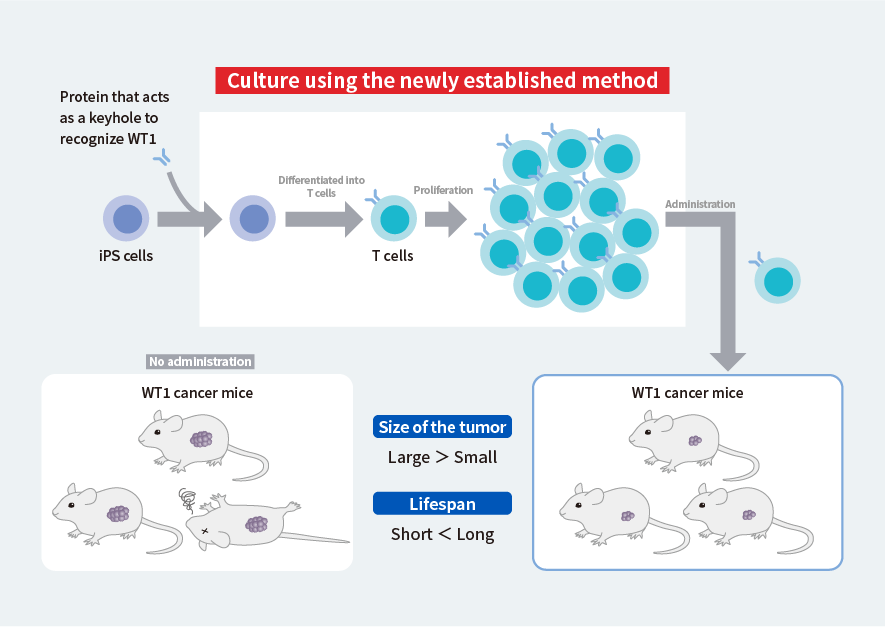 Figure 2. Experiment to confirm functioning of T cells generated using the new method
Figure 2. Experiment to confirm functioning of T cells generated using the new method
We also tried using the same method to produce the T cells used for CAR-T therapy (CAR-T cells). We named these cells iCART cells, and investigation of their function in mice transplanted with leukemia cells showed longer lifespan compared to similar mice not treated with iCART cells.
We have thus confirmed that T cells can be safely and efficiently obtained from iPS cells with the method we have established, and that these T cells can be used for the treatment of cancer. There is a possibility of rejection, since the T cells derived from iPS cells do not come from the patient’s own body. Nonetheless, we could avoid the rejection, for example, by producing T cells from the type of iPS cells that suit the patient, allowing the advantages of being able to prepare large quantities of T cells in a short time to be used.
We intend to continue this research to contribute to the development of cancer therapies that use T cells.